
| RELATED INFO |
| * Center for Structural Biology |
| * Purdue Department of Biological Sciences |
| * Purdue College of Science |
| * Rossmann lab movies |

October 19, 2007
Purdue breaks ground on Hockmeyer Hall
WEST LAFAYETTE, Ind. - |
"Just as astronomers look out into the vast expanse of space to discover characteristics of stars and planets, structural biologists look inside living organisms to determine characteristics of viruses, proteins and the tiny elements of life," said Purdue President France A. Córdova.
"Purdue is home to pioneers in this field. Professor Michael Rossmann and his group were the second team in the world to map a virus, and the first to model a common cold virus. Just as astronomers require better telescopes to see farther into space, structural biologists require better microscopes to see smaller structures. The development of new techniques and new technology is critical to advancing the field. This new building will give Purdue's renowned structural biology group the space to continue to be at the forefront of this field."
 |
The $30 million, 65,690-square-foot building will provide Purdue's Center for Structural Biology research group. The group currently is housed in the basement of Lilly Hall.
Hockmeyer Hall will be located adjacent to Discovery Park at Harrison Street and Martin Jischke Drive and is named for Wayne T. Hockmeyer and his wife, Mary, who gave $5.3 million toward its construction. The groundbreaking is part of a two-week celebration leading up to Purdue's homecoming on Oct. 27. The events focus on ways Purdue is improving education and the quality of life in Indiana.
"Structural biology has become one of the most promising fields in science over the past several decades," said Wayne Hockmeyer, Purdue alumnus and founder of biotechnology company MedImmune. "The contributions to fundamental science and medicine made by Purdue's structural biology group are unsurpassed by any other research team. This new building will provide these talented researchers with the space and tools necessary to continue to lead the field. Their work is key to prevention and treatment of widespread disease."
 |
The facility was made possible by $16 million in gifts and is scheduled for completion in the fall of 2009. The building will include eight specialized labs and eight general labs for work in the areas of protein production, cell and virus culture, large molecule crystallization, X-ray diffraction, nuclear magnetic resonance spectroscopy, electron microscopy, and analytical and biophysical instrumentation.
In addition, the building will house 16 offices for structural biology faculty, 33 offices for students and staff, and three conference rooms.
The Science Women of Purdue alumnae group became the first group of women in Purdue's history to raise funds for a named space on the West Lafayette campus. The group gave $300,000 for the Hockmeyer Hall project and will name a laboratory space to honor the women who came before them and to inspire future generations. The group will continue to support diversity initiatives within the College of Science.
"There is a rich history of women in science that continues today at Purdue and is reflected in our administration, faculty and alumnae," said Jeffrey S. Vitter, the Frederick L. Hovde Dean of the College of Science. "Just this year, President George W. Bush awarded alumna Rita R. Colwell the National Medal of Science for her accomplishments in the study of the agent that causes cholera. The Science Women of Purdue named laboratory space recognizes the pivotal role and fundamental breakthroughs women have contributed to the field."
Structural biology examines the basic building blocks of biological materials - molecules and atoms - and how they are put together.
"Seeing is, in many ways, understanding how things work," said Richard Kuhn, head of the Department of Biological Sciences. "Discovering how molecules are put together gives tremendous insight as to how they might function and provides a greater understanding of biological processes."
Purdue's Center for Structural Biology group studies a diverse group of problems, including cellular signaling pathways, RNA catalysis, bioremediation, molecular evolution, viral entry, viral replication and viral pathogenesis. Researchers use a combination of X-ray crystallography, electron cryomicroscopy, NMR spectroscopy, and advanced computational and modeling tools to study these problems.
One of the areas that needs a large amount of space and requires carefully controlled conditions is electron microscopy. Purdue's structural biology group has five electron microscopes, three of which are advanced high-end cryoelectron microscopes that allow researchers to see nearly down to the molecular level. Each microscope takes up a small room, and the slightest vibrations can disturb the images produced. The new building will allow the group the space needed to house the large equipment necessary to advance structural biology, Kuhn said.
 |
"We have historically been very successful, and this new building will move us to the next level," he said. "The building will allow us to group our high-end instruments in such a way that researchers can easily move between laboratories and branch out into research techniques they may not have used. The building will facilitate the interaction and collaboration necessary to keep Purdue at the forefront of this field."
Purdue's structural biology group has had many breakthroughs during the past 40 years, including fundamental insights into how important groups of human viruses infect cells, build themselves and are recognized by the human body. Also, the group has achieved important breakthroughs in understanding the structure of membrane proteins, which are the gateways into and out of cells, Kuhn said.
Some recent examples of the group's work include:
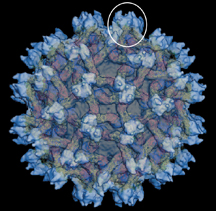
* A team including Rossmann, Kuhn and Timothy Baker mapped the structure of the dengue virus, knowledge that could prove important to the development of antiviral drugs. Dengue, a relative of West Nile virus and yellow fever, is spread by mosquitoes and kills more than 24,000 people annually. The group also determined the structure of the immature dengue particle while still within its cellular host, which could shed light on the virus' development process.
* Rossmann's team analyzed the structure of the baseplate of the T4 virus, which commonly infects E. coli bacteria. The baseplate is a complex structure made of 16 types of proteins that allows T4 to attach itself to the surface of E. coli. The team also obtained clearer pictures of how the baseplate alters its shape as T4 prepares to pierce E. coli's cell membrane. The team took images of the baseplate from different moments in the process, which resembles a flower opening, and transformed them into a brief animated movie, helping scientists understand how infection occurs.
* Kuhn and Rossmann's team determined the structure of the West Nile virus using cryo-electron microscopy and determined the orientation of the major surface proteins in the viral particle. Because these proteins allow the virus to invade a host cell, the research could be a step forward in combating the deadly mosquito-borne disease. In addition, the team found the precise location where antibodies bind to the virus and were able to offer a theory of how the antibody disarms the virus - crucial information for the development of a vaccine.
* William Cramer's group, working on structure-function of proteins embedded in biological membranes, obtained a molecular-scale picture of one of the major membrane protein complexes used by plants to convert sunlight to chemical energy. The effort revealed information not only about a process crucial to life on Earth, but also about how cells handle and distribute energy. The group has also obtained the most detailed structures available of cellular receptors used for uptake of vitamins and nutrients.
* David Sanders' research team replaced the genetic material inside the Ross River virus with helpful genetic material, enabling them to alter the liver cells of living mice without producing the harmful side effects that have accompanied the use of other viruses. Sanders' team also has redesigned the shell of Ebola, transforming the feared virus into a benevolent workhorse for gene therapy. And, as one of the first gene bearers that can be inhaled, the transformed virus might prove valuable in fighting lung disease.
* Carol Post's group has found the likely reason why a WIN compound - a prototype drug for curing colds - is showing so much promise. The flexible molecule's structure may allow it to shimmy inside the proteins that form the virus' outer shell and alter them to the point where the virus cannot complete the infection process.
* Jue Chen's group studies the process by which special proteins, called ABC proteins, open and close pathways into cells - permitting or denying materials entrance into the cell. This opening and closing is an integral part of the metabolic process and could be applied to drug delivery and cancer treatment.
Wayne and Mary Hockmeyer of Bethesda, Md., both grew up in Evansville, Ind. He earned his bachelor's degree in entomology from Purdue in 1966 and his doctorate from the University of Florida in 1972. Purdue awarded him an honorary doctorate in 2002. He returned that same year to participate in the Old Master's program, which allows current Purdue students to interact with past graduates to gain perspective on confronting challenges in their careers. Mary Hockmeyer earned her doctorate in human development at the University of Maryland in 1990.
After three months in his first job at Dow Chemical Co. in Michigan, he was commissioned in the Army, and, following airborne and special forces training, was sent to Vietnam in 1968 with the 5th Special Forces Group. The Army assisted with Hockmeyer's return to the University of Florida, where he earned his doctorate. He rose to the rank of lieutenant colonel and, during his 20-year military career, authored many research papers with particular emphasis on the development of malaria vaccines. He also was awarded the Legion of Merit, Bronze Star, Meritorious Service medal and the Army Commendation medal. The Legion of Merit and Meritorious Service medals were each separately awarded twice.
Following his military career, including the last six years as chairman of the Department of Immunology at the Walter Reed Army Institute of Research, Hockmeyer founded MedImmune Inc. in 1988 and served as president and CEO until 2000.
Hockmeyer was elected to serve on MedImmune's board of directors in 1988 and became chairman of the board of directors in 1993. The company developed and now markets Synagis®, an FDA-approved monoclonal antibody to prevent an infectious disease, and FluMist®, a live attenuated intranasal influenza vaccine. MedImmune generated more than $1 billion in annual revenue in 2006 and invested more than $438 million in research and development. The company has approximately 3,000 employees worldwide and was acquired by AstraZeneca plc in June 2007 for approximately $15.6 billion.
In addition to his duties as MedImmune's chairman, he also served as president of MedImmune Ventures Inc., the company's venture capital subsidiary, which was launched in 2002. He is a member of the Maryland Economic Development Commission and the Governor's Workforce Investment Board and serves on the boards of directors of several public companies, including Baxter International Inc., GenVec Inc., Idenix Pharmaceuticals Inc. and Middlebrook Pharmaceutical Corp.
Writer: Elizabeth Gardner, (765) 494-2081, ekgardner@purdue.edu
Sources: Jeffrey S. Vitter, (765) 494-1730, sciencedean@purdue.edu
Richard Kuhn, (765) 494-4407, kuhnr@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
PHOTO CAPTION:
Purdue University broke ground Friday (Oct. 19) on Wayne T. and Mary T. Hockmeyer Hall, a $30 million facility for the university's structural biology group. Wayne T. Hockmeyer, a Purdue alumnus and founder of biotechnology company MedImmune, and his wife, Mary T. Hockmeyer, gave $5.3 million toward the building construction. Pictured, from left, at the ceremony are: Richard Kuhn, head of the Department of Biological Sciences; Jeffrey S. Vitter, the Frederick L. Hovde Dean of the College of Science; Wayne and Mary Hockmeyer; and Purdue President France A. Córdova. (Purdue News Service photo/David Umberger)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2007/hockmyer-groundbreak.jpg
IMAGE CAPTION:
This image shows the immature dengue particle. Dengue is a mosquito-borne pathogen that kills more than 24,000 people in the world annually. A team including Purdue's Michael Rossmann and Richard Kuhn has solved the structure of the immature dengue virus, which is related to West Nile virus and yellow fever. The new Hockmeyer Hall of Structural Biology will allow Rossmann and Kuhn to further such work important to developing antiviral medicines. (Purdue University computer illustration)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2007/hockmeyer-research.jpg
To the News Service home page
If you have trouble accessing this page because of a disability, please contact Purdue News Service at purduenews@purdue.edu.

 *
*  *
*