

March 27, 2008
Findings reveal how dengue virus matures, becomes infectious
WEST LAFAYETTE, Ind. -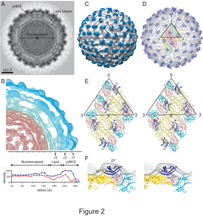 |
The findings pertain to all viruses in the family of flaviviruses, which includes a number of dangerous insect-borne diseases such as dengue, West Nile, yellow fever and St. Louis encephalitis. Dengue is prevalent in Southeast Asia, Central America and South America. The virus, which is spread by mosquitoes, infects more than 50 million people annually, killing about 24,000 each year, primarily in tropical regions.
The researchers detailed critical changes that take place as the virus is assembled and moves from the inner to the outer portions of its host cell before being secreted so that it can infect other cells. Virus particles are exposed to progressively less acidic conditions as they traverse this "secretory pathway," and this changing acidity plays a vital role in the maturation of the virus.
"This is possibly the most detailed understanding of how any virus matures," said Michael Rossmann, the Hanley Distinguished Professor of Biological Sciences.
The research is a collaboration of work in two Purdue laboratories, one operated by Rossmann and other operated by Jue Chen, an associate professor of biological sciences. They led the research with I-Mei Yu, a postdoctoral research associate working with Chen; and Long Li, a doctoral student working with Rossmann.
Findings are detailed in two back-to-back research papers appearing Friday (March 28) in the journal Science. The papers' co-authors include Yu, Li, Rossmann, Chen and Richard J. Kuhn, a professor and head of Purdue's Department of Biological Sciences.
Whereas the pathway for viruses entering new host cells has been studied extensively, the route for viruses moving out of their original host cells is not well-understood, Rossmann said.
"These two papers concern that route and compare the differences between both pathways," he said.
The virus moves through compartments inside the cell called the endoplasmic reticulum and the trans-Golgi network. While immature, virus particles are incapable of fusing with cell membranes, preventing them from infecting their own host cells and ensuring their maturation. Once mature, however, the virus is able to fuse to cell membranes, a trait that enables virus particles to infect new host cells, Chen said.
"There are many membranes in this trans-Golgi network, so the immature virus is always surrounded by membranes," Chen said. "In fact, the environment of the secretory pathway is very similar to what the virus encounters while it enters and infects a new host cell. So the question is, why doesn't the virus fuse to membranes on the way out?"
The researchers have examined the crucial role played by the changing acidity as the immature virus travels through the compartments.
"This change in acidity was already known, but its impact on the maturation process was not known until these new findings," Rossmann said.
As a virus particle matures along the pathway through the host cell, it changes the protein structure, or "conformation," in its outer shell.
Yu mimicked the trans-Golgi network environment in test tubes, enabling the researchers to study the virus's changing structure with increasing acidity.
The surface of each virus particle contains 180 copies of a component made of two linked proteins called precursor membrane protein and envelope protein.
The precursor membrane protein prevents the immature virus from fusing with membranes by covering an attachment site in the envelope protein. During maturation, an enzyme called furin snips the connection between the two proteins, eventually exposing the envelope protein site and enabling the virus to fuse with membranes.
Yu learned, however, that the precursor membrane protein remains in place until the virus is ready to exit the original host cell. The researchers used a technique called cryoelectron microscopy to gain a more detailed view of the virus.
"So, the precursor membrane protein is retained on the virus surface even after the enzyme detaches the two proteins," Chen said. "This is a critical step because the virus is ready to mature but still is incapable of fusing with membranes until after it exits its own cell."
The researchers also determined that the environment must be acidic before the enzyme will snip the two proteins, and they examined the structure to learn specifically why the increased acidity is needed.
Li used fruit fly cells to produce large quantities of the linked proteins so that researchers could study them with a method called X-ray crystallography. Using crystallography, the researchers were able to visualize and study the combined structure of the precursor membrane and envelope proteins.
"Having a better understanding of this structure will enable us to learn why the immature form does not fuse with membranes," Rossmann said. "Ultimately, researchers might want to find ways to treat or prevent viral infections, but in order to do that we first have to learn how viruses work, how they mature and initiate infection."
To produce the complex of the two proteins, Li first had to replace the insoluble "transmembrane region" of the protein with a soluble segment, a step essential for using the fruit fly cells to manufacture the proteins. He also had to mutate the protein to remove sites where furin normally attaches, preventing the proteins from being snipped apart.
The precursor membrane protein is about as wide as 50 nanometers, or billionths of a meter, and the envelope protein is about 3 nanometers, or nearly atomic-scale. A nanometer is about the size of 10 hydrogen atoms strung together.
The research has been funded primarily by the National Institutes of Health. Rossmann's and Chen's research laboratories are affiliated with Purdue's Markey Center for Structural Biology.
One of the papers was authored by Li, postdoctoral research associate Shee-Mei Lok, Yu, graduate student Ying Zhang, Kuhn, Chen and Rossmann. The other paper was authored by Yu, research scientist Wei Zhang, electron microscopist Heather A. Holdaway, Li, postdoctoral research associate Victor A. Kostyuchenko, electron microscopist Paul R. Chipman, Kuhn, Rossmann and Chen.
Future research may focus on determining the virus's changing structure in greater detail.
Writer: Emil Venere, (765) 494-4709, venere@purdue.edu
Sources: Michael Rossmann, (765) 494-4911, mr@purdue.edu
Jue Chen, (765) 496-3113, chenjue@purdue.edu
Richard J. Kuhn, (765) 494-1164, kuhnr@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Note to Journalists: Copies of the research paper are available by contacting the Science press package team at scipak@aaas.org, (202) 326-6440. I-Mei Yu pronounces her name Eee-May-You, and Long Li's last name is pronounced Lee.
IMAGE CAPTION:
This composite shows an image of the dengue virus, top left, taken with cryoelectron microscopy, and, to the right of that image are reconstructions of how virus particles mature as they move through their host cells. Purdue biologists have determined why the virus undergoes structural changes as it matures in host cells and how the changes are critical for enabling the virus to infect new host cells. Other elements of the composite show structural details of the virus and a vital component made of two linked proteins called precursor membrane protein and envelope protein. (Purdue University, Department of Biological Sciences)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2008/rossmann-mature.jpg
Structure of the immature dengue virus at low pH primes proteolytic maturation
I-Mei Yu, Weki Zhang, Heather A. Holdaway, Long Li, Victor A. Kostyuchenko, Paul R. Chipman, Richard J. Kuhn,
Michael G. Rossmann and Jue Chen
Intracellular cleavage of immature flaviviruses is a critical step in assembly that generates the membrane fusion potential of the E glycoprote4in. Using cryoelectron microscopy we show that the immature dengue particles undergo reversible conformational change at low pH which renders them accessible to furin cleavage. At pH 6.0, the E proteins are arranged in a herringbone pattern with the pr peptides docked onto the fusion loops, a configuration similar to that of the mature virion. After cleavage the dissociatoin of pr is pH-dependent, suggesting that in the acidic environment of the trans-Golgi network pr is retained on the virion to prevent membrane fusion. These results suggest a mechanism by which flaviviruses are processed and stabilized in the host cell secretory pathway.
The Flavivirus Precursor Membrane-Envelope Protein Complex: Structure and Maturation
Long Li, Shee-Mei Lok, I-Mei Yu, Ying Zhang, Richard J. Kuhn, Jue Chen and Michael G. Rossmann
Department of Biological Sciences, Purdue University
Many viruses go through a maturation step in the final stages of assembly before being transmitted to another host. The magturation process of flaviviruses is directed by the proteolytic cleavage of the precursor membrane protein (prM), turning inert virus into infectious particles. We have determined the crystal structure of a recombinant protein in which the dengue virus prM is linked to the envelope glycoprotein E. The structure represents the prM-E heterodimer and fits well into the cryo-electron microscopy density of immature virus at neutral pH. The pr peptide (beta symbol)-barrel structure covers the fusion loop in E, preventing fusion with host cell membranes. The structure provides a basis for identifying the stages of its pH-directed, conformational metamorphosis during maturation, ending with release of pr when budding from the host.
To the News Service home page
If you have trouble accessing this page because of a disability, please contact Purdue News Service at purduenews@purdue.edu.
