Regenerative biomaterial innovator GeniPhys awarded NSF SBIR Phase IIB grant
Company founded by Purdue University researcher expected to commercialize wound management product in Q2 2025

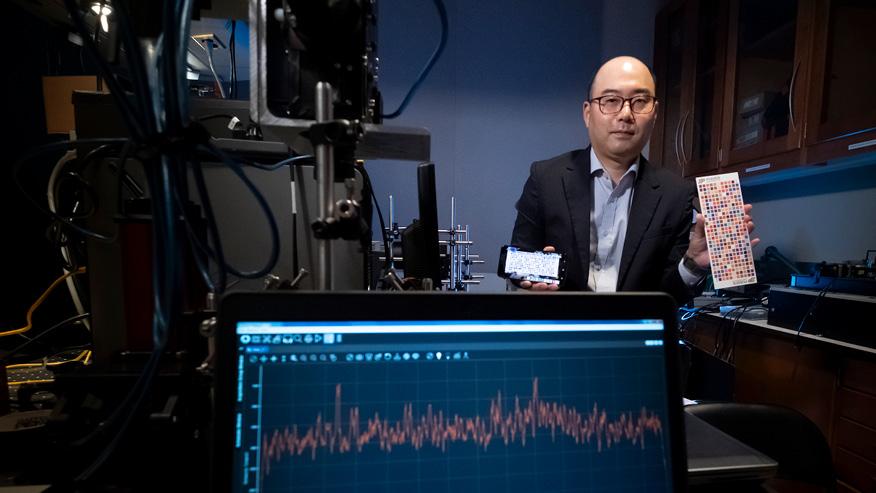
Sherry Harbin, founder and chief technology officer of GeniPhys and Purdue University researcher, shows a high-strength collagen sheet fabricated from the proprietary collagen polymer technology that GeniPhys is commercializing. GeniPhys has received a $500,000 SBIR Phase IIB grant from the National Science Foundation to support regulatory and commercial readiness of its flagship product. (Purdue Research Foundation photo/Vincent Walter)
INDIANAPOLIS — GeniPhys, a preclinical-stage company developing regenerative collagen polymeric biomaterials for soft tissue restoration, has been awarded a $500,000 National Science Foundation (NSF) Small Business Innovation Research (SBIR) Phase IIB supplemental funding grant to support regulatory and commercial readiness of its flagship product, Collymer Self-Assembling Scaffold (SAS). The Phase IIB grant supplements a nearly $1 million NSF SBIR Phase II grant awarded to GeniPhys in 2022 and will support completion of manufacturing scalability and necessary testing to achieve 510(k) clearance of Collymer SAS for the wound care market.
“Hard-to-heal soft tissue defects and voids due to injury, disease, congenital birth defects or tumor removal are a major burden to both patients and the health care system,” said Andy Eibling, GeniPhys CEO. “GeniPhys is answering a longtime need for novel options for rapid and effective soft tissue restoration. This grant helps bring us into the homestretch of offering patients the potential of a more cost-effective treatment with more predictable outcomes and shorter healing times.”
Collymer SAS is a novel flowable collagen biomaterial based on a validated and proprietary technology platform that immediately restores tissue continuity via a rapid-forming, self-assembling collagen scaffold. Unlike traditional implantable materials, this collagen scaffold promotes regenerative remodeling — facilitating integration, cellularization and restoration of tissue characteristics without causing an inflammatory response. Its structural and signaling characteristics replicate those of natural collagen for faster healing and restoration of tissue defects and voids affecting soft tissues such as skin, breast, skeletal muscle and adipose.
While Collymer SAS has a wide range of applications across various surgical specialties, GeniPhys will initially enter the advanced wound care market, where more than 10 million patients are affected with nonhealing, chronic wounds. Simultaneously, GeniPhys will pursue additional indications, including breast-conserving surgery (lumpectomy) where preclinical study results have shown Collymer SAS excels as a first-in-kind regenerative breast tissue filler with potential to improve both oncologic and cosmetic outcomes.
Collymer SAS was developed by GeniPhys founder and chief technology officer Sherry Harbin, professor of biomedical engineering and courtesy professor of basic medical sciences at Purdue University. The patented technology is licensed to GeniPhys by the Purdue Innovates Office of Technology Commercialization and is the cornerstone of the company’s broad intellectual property portfolio. Harbin also is part of Purdue’s collaborative One Health initiative.
About GeniPhys
GeniPhys™ is a preclinical-stage company developing first-in-class restorative biomaterials from Collymer™, a highly purified, self-assembling collagen protein building block. When interfaced with the body, Collymer materials uniquely replicate human collagen scaffold and support tissue remodeling without an inflammatory response, providing increased opportunities for improved patient outcomes, shorter healing times and the potential for more cost-effective treatments.
The patented Collymer technology is a next generation engineered collagen biopolymer that can be customized into multiple material formats, enabling applications across numerous medical and surgical specialties. The GeniPhys vision is to become a global manufacturer and supplier of Collymer and implantable bioregenerative materials — leading the industry in innovative solutions for the rapidly growing demands for advanced wound care management and regenerative medicine.
About Purdue Innovates Office of Technology Commercialization
The Purdue Innovates Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university’s academic activities through commercializing, licensing and protecting Purdue intellectual property. In fiscal year 2024, the office reported 145 deals finalized with 224 technologies signed, 466 invention disclosures received, and 290 U.S. and international patents received. The office is managed by the Purdue Research Foundation, a private, nonprofit foundation created to advance the mission of Purdue University. Contact otcip@prf.org for more information.
Media contact: Sue Goin, sue.goin@sapphire-com.com, 317-402-8690