February 13, 2020
New technology for pathogen detection driven by lasers
 Purdue innovators have developed a lanthanide-based assay coupled with a laser that can be used to detect toxins and pathogenic E. coli in food samples, water and a variety of industrial materials. (Image provided)
Download image
Purdue innovators have developed a lanthanide-based assay coupled with a laser that can be used to detect toxins and pathogenic E. coli in food samples, water and a variety of industrial materials. (Image provided)
Download image
Technology combines innovative assays with laser pulses
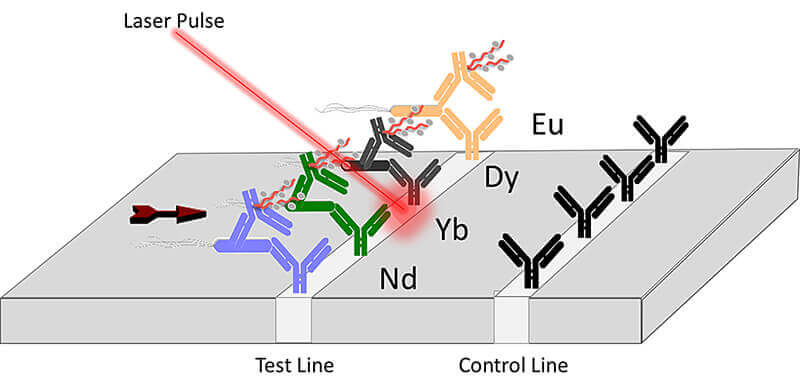
WEST LAFAYETTE, Ind. – Researchers at Purdue University have been working to develop new technologies to help stop the spread of foodborne illnesses, which kill 3,000 people a year, by detecting them more efficiently. They have developed a lanthanide-based assay coupled with a laser that can be used to detect toxins and pathogenic E. coli in food samples, water and a variety of industrial materials.
The two key features of the new technology are the incorporation of lanthanides and simple lateral flow paper-based assays. The Purdue team created a method for combining different heavy metals that when linked to antibodies can detect multiple agents in a single analysis. The Purdue team’s work is published in the January edition of Analytical and Bioanalytical Chemistry.
“Our goal was to incorporate easily detectable elements into a paper-based assay which is low-cost and effective,” said J. Paul Robinson, the SVM Professor of Cytomics in Purdue’s College of Veterinary Medicine and a professor of biomedical engineering in Purdue’s College of Engineering. “Designing a technology that is both low-cost but also accurate and can detect multiple antigens simultaneously was a critical factor in our decision to work on this problem.”
The innovators worked with the Purdue Research Foundation Office of Technology Commercialization to patent the technology in the United States and in Europe. They are looking for partners. For more information, contact Dipak Narula of OTC at dnarula@prf.org and reference track code 2019-ROBI-68413.
“We are very excited about the acceptance of the intellectual property as this will enhance the possibility of finding commercial partners,” Robinson said. “The potential for moving this to handheld, field deployable use is something we see in the future.”
The group is evaluating the potential for fully portable use that would allow field use in virtually any environment.
The approach uses a high-powered laser pulse to obliterate a sample, while simultaneously collecting the spectral signature of the resultant emission. These signals are then compared with a database that translates the signals into an identification of the toxin or pathogen.
The work presented in this paper shows the proof of principle and is the basis for significant expansion of the studies. What makes the technology effective is the linking of antibodies to different heavy metal tags. This creates a unique fingerprint of atomic signatures that can be used to determine if any particular pathogen of interest in present in a sample.
The U.S. Department of Agriculture Agricultural Research Service (ARS) and Center for Food Safety Engineering (CFSE) provided funding for the technology research in addition to Hatch Funds, which support agricultural research at land-grant institutions across the U.S.
About Purdue Research Foundation Office of Technology Commercialization
The Purdue Research Foundation Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university's academic activities through commercializing, licensing and protecting Purdue intellectual property. The office recently moved into the Convergence Center for Innovation and Collaboration in Discovery Park District, located on the west side of the Purdue campus. The office is managed by the Purdue Research Foundation, which received the 2019 Innovation and Economic Prosperity Universities Award for Place from the Association of Public and Land-grant Universities. The Purdue Research Foundation is a private, nonprofit foundation created to advance the mission of Purdue University. Contact otcip@prf.org for more information.
Writer: Chris Adam, 765-588-3341, cladam@prf.org
Source: J. Paul Robinson, wombat@purdue.edu
ABSTRACT
Detection of E. coli labeled with metal-conjugated antibodies using lateral-flow assay and laser-induced breakdown spectroscopy
Carmen Gondhalekar, Eva Biela, Bartek Rajwa, Euiwon Bae, Valery Patsekin, Jennifer Sturgis, Cole Reynolds, Iyll-Joon Doh, Prasoon Diwakar, Larry Stanker, Vassilia Zorba, Xianglei Mao, Richard Russo and J. Paul Robinson
This study explores the adoption of laser-induced breakdown spectroscopy (LIBS) for the analysis of lateral-flow immunoassays (LFIAs). Gold (Au) nanoparticles are standard biomolecular labels among LFIAs, typically detected via colorimetric means. A wide diversity of lanthanide-complexed polymers (LCPs) are also used as immunoassay labels but are inapt for LFIAs due to lab- bound detection instrumentation. This is the first study to show the capability of LIBS to transition LCPs into the realm of LFIAs, and one of the few to apply LIBS to biomolecular label detection in complete immunoassays. Initially, an in-house LIBS system was optimized to detect an Au standard through a process of line selection across acquisition delay times, followed by deter- mining limit of detection (LOD). The optimized LIBS system was applied to Au-labeled Escherichia coli detection on a commercial LFIA; comparison with colorimetric detection yielded similar LODs (1.03E4 and 8.890E3 CFU/mL respectively). Optimization was repeated with lanthanide standards to determine if they were viable alternatives to Au labels. It was found that europium (Eu) and ytterbium (Yb) may be more favorable biomolecular labels than Au. To test whether Eu-complexed polymers conjugated to antibodies could be used as labels in LFIAs, the conjugates were successfully applied to E. coli detection in a modified commercial LFIA. The results suggest interesting opportunities for creating highly multiplexed LFIAs. Multiplexed, sensitive, portable, and rapid LIBS detection of biomolecules concentrated and labeled on LFIAs is highly relevant for applications like food safety, where in-field food contaminant detection is critical. The silicon photomultiplier (SiPM) for low light detection has many advantages when compared to existing photon counting detectors, such as high sensitivity, low cost, robustness, and compact hardware. To facilitate the use of SiPM as a portable, field deployable device, an electrical circuit was designed consisting of an amplifier, comparator, and microcontroller. In addition, a 3D printing was used to create a portable cradle for housing the SiPM. To evaluate its detection ability, a laser experiment and bioluminescent experiments, including Pseudomonas fluorescens M3A detection, E. coli O157:H7 PhiV10nluc lysogen detection, and a luminescence-based detection of E. coli O157:H7 in ground meat using the engineered luminescent-based reporter phage PhiV10nluc, were conducted. In the same experimental setting, our previously developed smartphone-based luminometer called the bioluminescent-based analyte quantitation by smartphone and a conventional photomultiplier tube-based benchtop luminometer were used to compare detection levels and applicability for supporting luminescent phage-based pathogen detection. Results showed that the SiPM provides better performance in terms of time to detection and SNR and could be used as the light detection component of the PhiV10nluc phage-based detection format.

