July 18, 2018
Metal too 'gummy' to cut? Draw on it with a Sharpie or glue stick, science says

The application of a permanent marker, glue or tape makes gummy metals such as aluminum, stainless steels, copper and tantalum much easier to cut for industrial applications. (Purdue University image/Erin Easterling)
WEST LAFAYETTE, Ind. — Your everyday permanent markers, glue sticks and packing tape may offer a surprisingly low-tech solution to a long-standing nuisance in the manufacturing industry: Making soft and ductile, or so-called "gummy" metals easier to cut.
What makes inks and adhesives effective isn't their chemical content, but their stickiness to the surface of any gummy metal such as nickel, aluminum, stainless steels or copper, researchers at Purdue University and the University of West Florida find in a study recently published in Physical Review Applied.
These adhesives help achieve a smoother, cleaner and faster cut than current machining processes, impacting applications ranging from the manufacturing of orthopedic implants and surgical instruments to aerospace components.
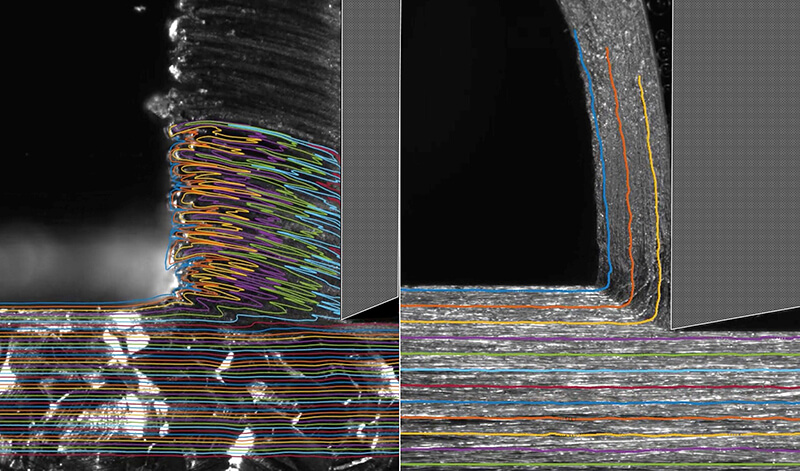 Purdue researchers have discovered a simple solution for cutting soft gummy metals (left) just as cleanly and easily as hard metals (right). (Purdue University image/Anirudh Udupa)
Download image
Purdue researchers have discovered a simple solution for cutting soft gummy metals (left) just as cleanly and easily as hard metals (right). (Purdue University image/Anirudh Udupa)
Download image
“A wide range of products rely on the machining of gummy metals. These could be something we use every day, such as the valve in a sink faucet, or something more critical like a compressor part in the jet engine of an airplane,” said James Mann, assistant professor of mechanical engineering at the University of West Florida and Purdue alumnus.
If a significant improvement can be made to the “machinability” of gummy metals or alloys – which is how well they cut, drill or grind – then there is potential to lower the cost of products, improve their performance or enable new and improved designs.
"Gummy metals characteristically deform in a very wiggly manner," said Srinivasan Chandrasekar, Purdue professor of industrial engineering. "This wiggly flow involves significant energy consumption, which means that these metals require more force to machine than even some hard metals. We needed to find a way to suppress this wiggly flow."
Getting rid of the wiggles means that the metal now tends to act more like a brittle ceramic or glass in the spot where it needs to be cut.
One well-known way to make the gummy metal brittle is by coating it with a suitable liquid metal, such as gallium in the case of aluminum. Liquid metals like these, however, tend to work too well; diffusing through the surface and causing the whole metal to crumble into a powder.
"This makes the metal being machined unusable," Chandrasekar said.
Other attempts met with limited success tended to be either toxic or result in tears and cracks on the machined surface. The researchers then began to explore other benign chemical media that would cut cleaner.
Marking with ink or attaching any adhesive on the metal's surface dramatically reduced the force of cutting without the whole metal falling apart, leaving a clean cut in seconds. The quality of the machined surface also greatly improved. Watch a YouTube video to see how at https://youtu.be/gjwPAgFAQUE.
Stickiness didn't initially stand out as a solution that permanent markers, glue sticks and tape have in common.
"We looked at the chemical ingredients of the permanent ink, isolated each of those on the metal's surface, and there was no noticeable effect," said Anirudh Udupa, lead author on the study and a postdoctoral researcher in Purdue's School of Industrial Engineering. "So we realized that it's not a particular chemical but the ink itself sticking to the metal through a physical adsorption mechanism."
 Purdue researcher Anirudh Udupa shows how marking a gummy metal's surface with an ink or adhesive dramatically reduces the force required to cut it. (Purdue University image/Erin Easterling)
Download image
Purdue researcher Anirudh Udupa shows how marking a gummy metal's surface with an ink or adhesive dramatically reduces the force required to cut it. (Purdue University image/Erin Easterling)
Download image
The Sharpie and adhesives also appeared to work on many gummy metals, regardless of the cutting tool.
"In hindsight, we can tell you why certain things weren't successful in previous work. It all comes back to the existence of this wiggly flow," said Koushik Viswanathan, Purdue postdoctoral researcher in industrial engineering. "Some people might have been trying to cut copper, for example, that was in the hard state rather than in the soft state."
To the researchers' knowledge, using permanent markers, glues or tape to make gummy metals easier to machine does not pose any environmental hazards.
Next, Chandrasekar's group will be assessing the degree of stickiness that works best for cutting gummy metals and exploring ways to advance the application of this technology into industrial practice.
This research is supported by the U.S. Army Research Office (W911NF-15-1-0591), the National Science Foundation (CMMI 1562470 and DMR 1610094) and the U.S. Department of Energy (DE-EE0007868).
Writer: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Sources: Srinivasan Chandrasekar, 765-494-3623, chandy@purdue.edu
Anirudh Udupa, audupa@purdue.edu
Koushik Viswanathan, kviswana@purdue.edu
James Mann, JBMann@uwf.edu
Note to Journalists: For a full-text copy of the paper, please contact Kayla Wiles, Purdue News Service, at wiles5@purdue.edu. A YouTube video is available at https://youtu.be/gjwPAgFAQUE, and other multimedia can be found in a Google Drive folder at https://goo.gl/wRQkVU. The materials were prepared by Erin Easterling, digital producer for the Purdue College of Engineering, 765-496-3388, easterling@purdue.edu.
ABSTRACT
Anirudh Udupa1, Koushik Viswanathan1, Mojib Saei1,
James B. Mann2, and Srinivasan Chandrasekar1
1Purdue University, West Lafayette, IN, USA
2University of West Florida, Pensacola, FL, USA
doi: 10.1103/PhysRevApplied.10.014009
Soft and highly strain hardening metals like iron, aluminum and tantalum, often called gummy, are notoriously difficult to cut. This is due to their tendency to exhibit redundant, unsteady plastic flow with large-amplitude folding, and which results also in macro-scale defects on the cut surface and large energy dissipation. In this work, we demonstrate that this difficulty can be overcome by merely coating the initial metal surface with common adhesive chemical media like glues and inks. Using high-speed in situ imaging, we show that the media act by coupling unsteady surface plastic flow modes with interface energetics - a mechanochemical action - thereby effecting a ductile to-brittle transition, locally. Consequently, the unsteady plastic flow with folding transitions to a periodic segmentation-type flow in the presence of the surface media, with near absence of defects on the cut surface and significantly lower energy dissipation (reduction of up to 80%). This mechanochemical effect is controllable and not material specific, with the chemical media demonstrating comparable efficacy across di↵erent metal systems. This makes it quite distinct from other well-known mechanochemical e↵ects, such as liquid metal embrittlement and stress corrosion cracking, that are both highly material-specific and catastrophic. An analytical model incorporating local flow dynamics, stability of dislocation emission and surface media energetics is found to correctly predict the onset of the plastic flow transition. The benign nature and simplicity of the media suggests wide-ranging opportunities for improving performance of cutting and deformation processes for metals and alloys in practical settings.

