August 15, 2018
Quantum material is promising ‘ion conductor’ for research, new technologies
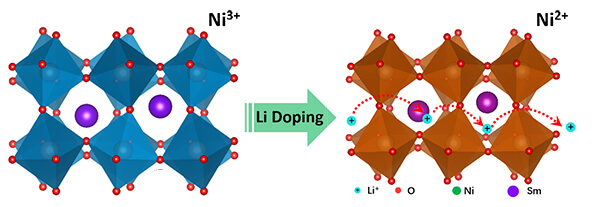 This graphic depicts new research in which lithium ions are inserted into the crystal structure of a quantum material called samarium nickelate, suggesting a new avenue for research and potential applications in batteries, “smart windows” and brain-inspired computers containing artificial synapses. (Purdue University image/Yifei Sun)
Download image
This graphic depicts new research in which lithium ions are inserted into the crystal structure of a quantum material called samarium nickelate, suggesting a new avenue for research and potential applications in batteries, “smart windows” and brain-inspired computers containing artificial synapses. (Purdue University image/Yifei Sun)
Download image
WEST LAFAYETTE, Ind. – Researchers have shown how to shuttle lithium ions back and forth into the crystal structure of a quantum material, representing a new avenue for research and potential applications in batteries, “smart windows” and brain-inspired computers containing artificial synapses.
The research centers on a material called samarium nickelate, which is a quantum material, meaning its performance taps into quantum mechanical interactions. Samarium nickelate is in a class of quantum materials called strongly correlated electron systems, which have exotic electronic and magnetic properties.
The researchers “doped” the material with lithium ions, meaning the ions were added to the material’s crystal structure.
The addition of lithium ions causes the crystal to expand and increases the material’s conduction of the ions. The researchers also learned that the effect works with other types of ions, particularly sodium ions, pointing to potential applications in energy storage.
Findings are detailed in a paper appearing this week in Proceedings of the National Academy of Sciences.
“The results highlight the potential of quantum materials and emergent physics in the design of ion conductors,” said Shriram Ramanathan, a Purdue University professor of materials engineering who is leading the research. “There is a lot of research now going on to identify solid-state ion conductors for building batteries, for example. We showed that this general family of materials can hold these ions, so we established some general principles for the design of these sorts of solid-state ion conductors. We showed that ions like lithium and sodium can move through this solid material, and this opens up new directions for research.”
Applying a voltage caused the ions to occupy spaces between atoms in the crystal lattice of the material. The effect could represent a more efficient method to store and conduct electricity. Such an effect could lead to new types of batteries and artificial synapses in “neuromorphic,” or brain-inspired, computers. Moreover, the ions remained in place after the current was turned off, a “non-volatile” behavior that might be harnessed for computer memory.
Adding lithium ions to the crystal structure also changes the material’s optical properties, suggesting potential applications as coatings for “smart windows” whose light transmission properties are altered when voltage is applied.
The research paper’s lead authors are Purdue materials engineering postdoctoral research associate Yifei Sun and Michele Kotiuga, a postdoctoral fellow in the Department of Physics and Astronomy at Rutgers University. The work was performed by researchers at several research institutions. A complete listing of co-authors is available in the abstract. To develop the doping process, materials engineers collaborated with Vilas Pol, a Purdue associate professor of chemical engineering and materials engineering, and Purdue graduate student Dawgen Lim.
The research findings demonstrated behavior related to the “Mott transition,” a quantum mechanical effect describing how the addition of electrons can change the conducting behavior of a material.
“As we add more electrons to the system the material becomes less and less conducting, which makes it a very interesting system to study, and this effect can only be explained through quantum mechanics,” Ramanathan said.
Kotiuga’s contribution to the work was to study the electronic properties of lithium-doped samarium nickelate as well as the changes to the crystal structure after doping.
“My calculations show that undoped samarium nickelate is a narrow-gapped semiconductor, meaning that even though it is not metallic, electrons can be excited into a conducting state without too much trouble,” she said. “As lithium is added to samarium nickelate the lithium ion will bind to an oxygen and an electron localizes on a nearby nickel-oxygen octahedron, and when an electron has localized on every nickel-oxygen octahedron the material is converted into an insulator. This is a rather counterintuitive result: the added electrons to the system make the material more insulating.”
The material’s crystal structure was characterized using a synchrotron-radiation light source research facility at Argonne National Laboratory.
The researchers had been working on the paper for about two years and plan to further explore the material’s quantum behavior and potential applications in brain-inspired computing.
The research was funded or otherwise supported by several sources, including the National Science Foundation, U.S. Department of Energy, the Canadian Light Source, U.S. Army Research Office, U.S. Air Force Office of Scientific Research and the U.S. Office of Naval Research.
Writer: Emil Venere
Media contact: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Source: Shriram Ramanathan, 765-496-0546, shriram@purdue.edu
ABSTRACT
Strongly correlated perovskite lithium-ion shuttles
Yifei Suna*, Michele Kotiugab*, Dawgen Lima, Badri Narayananc, Mathew Cherukarad, Zhen
Zhanga, Yongqi Dongd, Ronghui Koud, Cheng-Jun Sund, Qiyang Lue,f , Iradwikanari Waluyog,
Adrian Huntg, Hidekazu Tanakah, Azusa N. Hattorih, Sampath Gamagei, Yohannes Abatei, Vilas G. Polj, Hua Zhoud,
Subramanian KRS Sankaranarayanak, Bilge Yildize,f,l Karin M. Rabeb, Shriram Ramanathana
aSchool of Materials Engineering, Purdue University, West Lafayette, Indiana 47907, USA.
bDepartment of Physics and Astronomy, Rutgers, The State University of New Jersey, NJ 08854, USA
cMaterials Science Division, Argonne National Laboratory, Argonne, Illinois 60439, USA
dX-ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, Illinois 60439, USA
eLaboratory for Electrochemical Interfaces, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
fDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
gNational Synchrotron Light Source II, Brookhaven National Laboratory, Upton, NY 11973, US
hInstitute of Scientific and Industrial Research, Osaka University, Osaka, 567-0047, Japan
IDepartment of Physics and Astronomy, University of Georgia, Athens, Georgia 30602, USA
jDavidson School of Chemical Engineering, Purdue University, West Lafayette, Indiana 47907, USA
kCenter for Nanoscale Materials, Argonne National Laboratory, Argonne, Illinois 60439, USA 25
lDepartment of Nuclear Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
*These authors contributed equally to this work
Correspondence and requests for materials should be addressed to S.R. (email: shriram@purdue.edu)
Solid state ion shuttles are of broad interest in electrochemical devices, non-volatile memory, neuromorphic computing and bio-mimicry utilizing synthetic membranes. Traditional design approaches are primarily based on substitutional doping of dissimilar valent cations in a solid lattice, which has inherent limits on dopant concentration and thereby ionic conductivity. Here we demonstrate perovskite nickelates as Li-ion shuttles with simultaneous suppression of electronic transport via Mott transition. Electrochemically lithiated SmNiO3 (Li-SNO) contains a large amount of mobile Li+ located in interstitial sites of the perovskite approaching one dopant ion per unit cell. A significant lattice expansion associated with interstitial doping allows for fast Li+ conduction with reduced activation energy. We further present a generalization of this approach with results on other rare-earth perovskite nickelates as well as dopants such as Na+. The results highlight the potential of quantum materials and emergent physics in design of ion conductors.

