April 22, 2020
Microwaves power new technology for batteries, energy
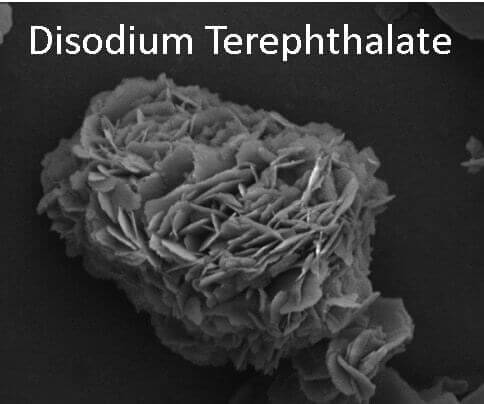 This image shows disodium terephthalate flowers produced from polyethylene terephthalate via microwave processing in two minutes. (Image provided)
Download image
This image shows disodium terephthalate flowers produced from polyethylene terephthalate via microwave processing in two minutes. (Image provided)
Download image
WEST LAFAYETTE, Ind. – New battery technology involving microwaves may provide an avenue for renewable energy conversion and storage.
Purdue University researchers created a technique to turn waste polyethylene terephthalate, one of the most recyclable polymers, into components of batteries.
“We use an ultrafast microwave irradiation process to turn PET, or polyethylene terephthalate, flakes into disodium terephthalate, and use that as battery anode material,” said Vilas Pol, a Purdue associate professor of chemical engineering who has worked with the Purdue Research Foundation Office of Technology Commercialization to develop several battery technologies. “We are helping to address the growth in the proliferation of renewable energy conversion and storage, which stems from the societal attention and increasing awareness of climate change and energy resource limitation.”
The Purdue team tried the approach with both lithium-ion and sodium-ion battery cells. They worked with researchers from the Indian Institute of Technology and Tufts University. The battery technology is presented in the journal ACS Sustainable Chemistry & Engineering.
Pol said that while lithium-ion technology is currently dominating both the portable electronics and electric vehicles market, sodium-ion battery research also has gained significant attention due to its low cost and appealing electrochemical performance in grid applications.
“The applicability of the microwave technique on organic reactions has gained attention in recent times due to its advantage of the rapid reaction process,” Pol said. “We have accomplished the complete conversion of PET to disodium terephthalate within 120 seconds, in a typical household microwave setup.”
Pol said the materials used in the Purdue technology are low-cost, sustainable and recyclable.
For more information on licensing and other opportunities with Pol’s battery technologies, contact OTC at otcip@prf.org.
About Purdue Research Foundation Office of Technology Commercialization
The Purdue Research Foundation Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university's academic activities through commercializing, licensing and protecting Purdue intellectual property. The office recently moved into the Convergence Center for Innovation and Collaboration in Discovery Park District, adjacent to the Purdue campus. The office is managed by the Purdue Research Foundation, which received the 2019 Innovation and Economic Prosperity Universities Award for Place from the Association of Public and Land-grant Universities. The Purdue Research Foundation is a private, nonprofit foundation created to advance the mission of Purdue University. Visit the Office of Technology Commercialization for more information.
Writer: Chris Adam, 765-588-3341, cladam@prf.org
Source: Vilas Pol, vpol@purdue.edu
ABSTRACT
Rapid Upcycling of Waste Polyethylene Terephthalate (PET) to Energy Storing Disodium Terephthalate Flowers with DFT Calculations
Sourav Ghosh, Maxim A. Makeev, Zhimin Qi, Haiyan Wang, Nav Nidhi Rajput, Surendra K. Martha and Vilas G. Pol
In this work, we report an efficient synthesis approach of disodium terephthalate and its application as a potential battery anode material. Disodium terephthalate is upcycled from waste PET flakes with the aid of ultrafast microwave irradiation process within 2 minutes. The phase and chemical purity of the as-synthesized disodium terephthalate is confirmed by X-ray diffraction, Fourier-transform infrared spectroscopy, and nuclear magnetic resonance spectroscopy. The electrochemical behavior of this low-cost, environmentally benign small organic molecule is studied in Li- and Na-ion cells. The density functional theory-based calculations are performed to get insights into specifics of electronic properties of Li- and Na-ion cells and rationalize the differences in behavior for the two systems. The delithiation potential of disodium terephthalate anode is found to be approximately 0.65 V higher than the desodiation potential. The disodium terephthalate- carbon black (Super P) composite electrode delivers discharge capacities of 182 and 224 mAh g-1 at the current density of 25 mA g-1, after 50 cycles in Li-ion and Na-ion cells, respectively. The better C-rate performance of the composite anode for Li-ion cell, as compared to Na-ion cell, is due to inferior mobility of Na-ions in the electrode material, which is largely defined by ion size.

