June 16, 2020
Wearable patch may provide new treatment option for skin cancer
 Chi Hwan Lee, a Purdue engineering innovator, developed a wearable patch to provide a new treatment option for skin cancer. (Image provided)
Download image
Chi Hwan Lee, a Purdue engineering innovator, developed a wearable patch to provide a new treatment option for skin cancer. (Image provided)
Download image
WEST LAFAYETTE, Ind. – Conventional melanoma therapies, including chemotherapy and radiotherapy, suffer from the toxicity and side effects of repeated treatments due to the aggressive and recurrent nature of melanoma cells.
Less invasive topical chemotherapies have emerged as alternatives, but their widespread uses have been hindered by both the painful size of the microneedles and the rapidly dissolving behavior of polymers used in the treatments.
Now, Purdue University innovators have created a novel wearable patch to help address the issues and provide an improved treatment experience for people with melanoma. The technology is published in the journal ACS Nano.
“We developed a novel wearable patch with fully miniaturized needles, enabling unobtrusive drug delivery through the skin for the management of skin cancers,” said Chi Hwan Lee, a Purdue assistant professor of biomedical engineering and mechanical engineering. “Uniquely, this patch is fully dissolvable by body fluids in a programmable manner such that the patch substrate is dissolved within one minute after the introduction of needles into the skin, followed by gradual dissolution of the silicon needles inside the tissues within several months.”
Lee said this gradual slow dissolution of the silicon nanoneedles allows for long-lasting and sustainable delivery of cancer therapeutics.
“The uniqueness of our technology arises from the fact that we used extremely small but long-lasting silicon nanoneedles with sharpened angular tips that are easy for their penetration into the skin in a painless and minimally invasive manner,” Lee said.
The Purdue innovators developed a novel design of bioresorbable silicon nanoneedles that are built on a thin, flexible and water-soluble medical film. The water-soluble film serves as a temporary holder that can be conformably interfaced with the soft, curvilinear surface of the skin during the insertion of the nanoneedles, followed by rapid, complete dissolution within a minute.
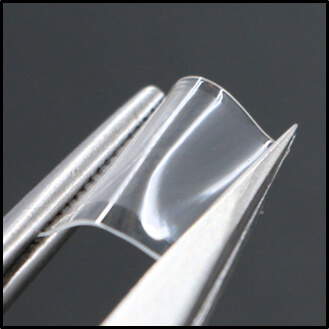 This image shows a bioresorbable wearable skin patch comprised with fully miniaturized silicon needles for the management of skin cancers. (Image provided)
This image shows a bioresorbable wearable skin patch comprised with fully miniaturized silicon needles for the management of skin cancers. (Image provided)
The surface of the nanoneedles is configured with nanoscale pores and provides a large drug loading capacity comparable to those using conventional microneedles.
Lee said the nanoneedles could deliver the chemotherapeutic drugs to target melanoma sites in a sustainable manner. The silicon nanoneedles are biocompatible and dissolvable in tissue fluids, such that they can be completely resorbed in the body over months in a harmless manner.
This work is supported by the Air Force Office of Scientific Research (AFOSR: FA2386-18-1-40171).
Lee said he started working on this type of technology after seeing his daughter express fearful thoughts about needles when receiving vaccinations.
The team worked with the Purdue Research Foundation Office of Technology Commercialization to patent this technology. The office recently moved into the Convergence Center for Innovation and Collaboration in Discovery Park District, adjacent to the Purdue campus.
The researchers are looking for partners to continue developing their technology. For more information on licensing and other opportunities, contact Patrick Finnerty of OTC at pwfinnerty@prf.org and mention track code 2020-LEE-68893.
Lee’s team worked with Yoon Yeo from Purdue’s College of Pharmacy and Dong Rip Kim at Hanyang University in South Korea to develop and test the technology.
About Purdue Research Foundation Office of Technology Commercialization
The Purdue Research Foundation Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university's academic activities through commercializing, licensing and protecting Purdue intellectual property. The office recently moved into the Convergence Center for Innovation and Collaboration in Discovery Park District, adjacent to the Purdue campus. In fiscal year 2019, the office reported 136 deals finalized with 231 technologies signed, 380 disclosures received and 141 issued U.S. patents. The office is managed by the Purdue Research Foundation, which received the 2019 Innovation and Economic Prosperity Universities Award for Place from the Association of Public and Land-grant Universities. In 2020, IPWatchdog Institute ranked Purdue third nationally in startup creation and in the top 20 for patents. The Purdue Research Foundation is a private, nonprofit foundation created to advance the mission of Purdue University. Contact otcip@prf.org for more information.
Writer: Chris Adam, 765-588-3341, cladam@prf.org
Source: Chi Hwan Lee, lee2270@purdue.edu
ABSTRACT
Bioresorbable, Miniaturized Porous Silicon Needles on Flexible Water-Soluble Backing for Unobtrusive, Sustained Delivery of Chemotherapy
Hyungjun Kim, Heung Soo Lee, Yale Jeon, Woohyun Park, Yue Zhang, Bongjoong Kim, Hanmin Jang, Baoxing Xu, Yoon Yeo, Dong Rip Kim and Chi Hwan Lee
Conventional melanoma therapies suffer from the toxicity and side effects of repeated treatments due to the aggressive and recurrent nature of melanoma cells. Less-invasive topical chemotherapies by utilizing polymeric microneedles have emerged as an alternative, but the sustained, long-lasting release of drug cargos remains challenged. In addition, the size of the microneedles is relatively bulky for the small, curvilinear, and exceptionally-sensitive cornea for the treatment of ocular melanoma. Here, we report a design of bioresorbable, miniaturized porous-silicon (p-Si) needles with covalently-linked drug cargos at doses comparable to those of conventional polymeric microneedles. The p-Si needles are built on a water-soluble film as a temporary flexible holder that can be intimately interfaced with the irregular surface of living tissues, followed by complete dissolution with saline solution within one minute. Consequently, the p-Si needles remain embedded inside tissues and then undergo gradual degradation allowing for sustained release of the drug cargos. Its utility in unobtrusive topical delivery of chemotherapy with minimal side effects is demonstrated in a murine melanoma model.

