
| RELATED WEB SITES |
| * Center for Analytical Instrumentation Home Page |
| * R. Graham Cooks Home Page |

January 2009
Purdue technology detects contaminant in milk products
WEST LAFAYETTE, Ind. -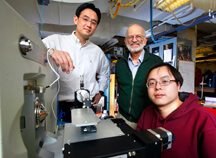 |
A research team at Purdue University created the analysis method to detect levels of melamine in the low parts-per-billion in milk and milk powder in about 25 seconds.
An estimated 50,000 Chinese children were sickened and several died after drinking the melamine-contaminated formula. Melamine, which is used in plastics, was deliberately added to the formula to artificially bump up apparent protein levels.
The chemical also was found in the contaminated pet food produced in China responsible for the deaths of a reported 8,500 dogs and cats in the United States in March 2007.
The U.S. Food and Drug Administration issued new guidelines in November limiting melamine in dairy products to 1 part-per-million or less.
"This situation created an immediate need for an analytical method that is highly sensitive, fast, accurate and easy to use," said R. Graham Cooks, Purdue's Henry B. Hass distinguished professor of chemistry, who led the team that developed the analysis method. "We took it as a challenge to use simpler instrumentation and to develop a faster method that allows the testing to be done on site and does not require pretreatment of samples."
 |
In addition to Cooks, the team includes Guangming Huang, a postdoctoral research associate, and Zheng Ouyang, an assistant professor of biomedical engineering. A paper detailing their work was published online in the journal Chemical Communications and will appear in the next issue of the journal.
"Even without direct contamination, trace amounts of melamine sometimes make their way into consumable products because melamine is used in manufacturing and is found in many packaging materials," Cooks said. "At trace levels, the chemical is not known to be a health threat and has been deemed safe by the FDA. Our analysis provides a way to determine whether the amounts present exceed safe levels."
The new method pairs mass spectrometry with a low-temperature plasma ionization probe technique.
Mass spectrometry is a commonly used analysis method known for its sensitivity and accuracy; however, most available mass spectrometers require that a sample be pretreated and remain in the controlled environment of a vacuum for analysis, Cooks said.
The Purdue team took advantage of the recent availability of new ambient ionization methods in which samples are examined in their native environment with little or no preparation, he said.
"Ambient ionization methods, such as the low-temperature plasma ionization we used, can greatly reduce the time-intensive and sometimes difficult requirements of mass spectrometers," Cooks said. "The experiment can be done in a high-throughput fashion, at a rate of two samples per minute. This method provides the sensitivity, specificity and the quantitative accuracy needed to meet the current urgent requirements for a simple and reliable melamine determination in complex mixtures."
The method directs a collection of charged particles, or plasma, onto the sample using a slow stream of helium or other gas. The plasma reacts with the sample and ionizes, or gives its charge to, some of the molecules in the sample. The charge gives the molecules mass and allows them to be identified by a mass spectrometer. The ionized sample molecules are then vacuumed into a mass spectrometer for analysis. Heating the sample assists in ionization, Cooks said.
During the experiments, a solid melamine-containing material was heated to approximately 340 degrees Fahrenheit. Liquids, such as milk, evaporated as they were heated and the residues were examined.
"There is a growing need in our society for detailed chemical information that calls for the special capabilities of mass spectrometers," Cooks said. "Researchers are working to make these devices faster, easier to use and more portable. Perhaps one day everyone will have a mass spectrometer to analyze whatever comes their way."
The Cooks and Ouyang groups have created several portable miniature mass spectrometers, including the most recent 9-pound Mini11, which is equipped with capabilities for ambient ionization. The team next will try to incorporate the new analysis method into the capabilities of the Mini11.
The Office of Naval Research Research Tools Program funded this research.
Cooks is co-founder of Purdue's Center for Analytical Instrumentation Development located at the Bindley Biosciences Center in Purdue's Discovery Park and also is affiliated with the Purdue Cancer Center.
Indianpolis-based Prosolia Inc. has commercialized the related Cooks' ambient ionization technology called desorption electrospray ionization, or DESI.
Writer: Elizabeth K. Gardner, (765) 494-2081, ekgardner@purdue.edu
Source: R. Graham Cooks, (765) 494-5263, cooks@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
PHOTO CAPTION:
From left, Purdue University researchers Zheng Ouyang, an assistant professor of biomedical engineering; R. Graham Cooks, the Henry Bohn Hass distinguished professor of chemistry; and Guangming Huang, a postdoctoral research associate, pose with the mass spectrometer used in a new method for detecting melamine in milk products. (Purdue University news service photo/Andy Hancock)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2009/cooks-melamine.jpg
PHOTO CAPTION:
Zheng Ouyang, an assistant professor of biomedical engineering at Purdue University, holds the Mini11, a miniature mass spectrometer. (Purdue University news service photo/Andy Hancock)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2009/mini-mass-spectrometer.jpg
High-throughput trace melamine
analysis in complex mixtures
Guangming Huang, Zheng Ouyang, and R. Graham Cooks
Ambient ionization using a low-temperature plasma (LTP) probe combined with tandem mass spectrometry (MS/MS) allows detection and quantitation of melamine in milk powder, whole milk and other products at levels down to low ppb in analysis times of a few tens of seconds.
To the News Service home page
