March 1, 2016
New direction urged to improve cancer nanotechnology
 |
|
The complex microenvironment of tumors is presenting a challenge in developing effective anticancer treatments that attempt to harness nanotechnology. Researchers are recommending pivotal changes in the field of cancer nanotechnology because experiments with laboratory animals and efforts based on current assumptions about drug delivery have largely failed to translate into successful clinical results. (Purdue University image/Bumsoo Han, Kinam Park, Murray Korc) |
WEST LAFAYETTE, Ind. – Researchers involved in a national effort to develop cancer treatments that harness nanotechnology are recommending pivotal changes in the field because experiments with laboratory animals and efforts based on current assumptions about drug delivery have largely failed to translate into successful clinical results.
The assessment was advanced in a perspective piece that appeared in the National Cancer Institute's Cancer Nanotechnology Plan 2015, a 10-year roadmap concerning the use of nanotechnology to attack cancer.
Researchers are trying to perfect "targeted delivery" methods using various agents, including an assortment of tiny nanometer-size structures, to selectively attack tumor tissue. However, the current direction of research has brought only limited progress, according to the authors of the article.
"The bottom line is that so far there are only a few successful nanoparticle formulations approved and clinically used, so we need to start thinking out of the box," said Bumsoo Han, a Purdue University associate professor of mechanical and biomedical engineering.
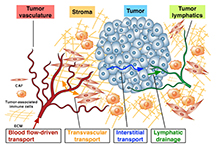
One approach pursued by researchers has been to design nanoparticles small enough to pass through pores in blood vessels surrounding tumors but too large to pass though the pores of vessels in healthy tissue. The endothelial cells that make up healthy blood vessels are well organized with tight junctions between them. However, the endothelial cells in blood vessels around tumors are irregular and misshapen, with loose gaps between the cells.
"We should realize that having a specific nanosize or functionality alone is not enough to guarantee good drug delivery to target tumors," said Kinam Park, a professor of pharmaceutics and Purdue's Showalter Distinguished Professor of Biomedical Engineering. "The tumor microenvironment is just too complex to overcome using this strategy alone."
The two authored the article with Murray Korc, the Myles Brand Professor of Cancer Research at the Indiana University School of Medicine.
The authors pointed out that research with laboratory mice has rarely translated into successful clinical results in humans, suggesting that a more effective approach might be to concentrate on research using in-vitro experiments that mimic human physiology. For example, one new system under development, called a tumor-microenvironment-on-chip (T-MOC) device, could allow researchers to study the complex environment surrounding tumors and the barriers that prevent the targeted delivery of therapeutic agents.
The approach could help drug makers solve a daunting obstacle: even if drugs are delivered to areas near the target tumor cells, the treatment still is hindered by the complex microenvironment of tumors.
"We used to think that if we just killed the tumor cell it would cure the cancer, but now we know it's not just the cancer cells alone that we have to deal with," Korc said. "There are a lot of different cells and blood vessel structure, making for a complex environment that supports the cancer cells."
An "extracellular matrix" near tumors includes dense collagen bundles and a variety of enzymes, growth factors and cells. For example, surrounding pancreatic tumors is a "stromal compartment" containing a mixture of cells called stromal cells, activated cancer-associated fibroblasts and inflammatory immune cells.
"Particularly for pancreatic cancer, the stromal tissue is much bigger than the tumor itself," Korc said.
In addition, a compound called hyaluronic acid in this stromal layer increases the toughness of tumor microenvironment tissue, making it difficult for nanoparticles and drugs to penetrate.
"It's dense, like scar tissue, so it's more difficult for drugs coming out of the blood vessel to diffuse through this tissue," Han said.
Another challenge is to develop water-soluble drugs to effectively deliver medicines.
"The cancer drugs need to be aqueous because the body resorbs them better, but a lot of the current chemotherapy drugs have low solubility and usually need different types of solvents to increase their solubility," Park said.
The T-MOC approach offers some hope of learning how to design more effective cancer treatments.
"Recent advances in tissue engineering and microfluidic technologies present an opportunity to realize in-vitro platforms as alternatives to animal testing," Park said. "Tumor cells can be grown in 3D matrices with other relevant stromal cells to more closely mirror the complexity of solid tumors in patients. The current ability of forming 3D-perfused tumor tissue needs to be advanced further to create an accurate tumor microenvironment."
Such a major shift in research focus could play a role in developing personalized medicine, or precision medicine, tailored to a particular type of cancer and specific patients. More effective treatment might require various "priming agents" in combination with several drugs to be administered simultaneously or sequentially.
"This kind of research currently involves a very large number of experiments, and it makes animal testing expensive and time consuming," Park said. "Moreover, small animal data have not been good predictors of clinical outcome. Thus, it is essential to develop in-vitro test methods that can represent the microenvironment of human tumors."
A group of researchers is working in the area of cancer nanotechnology at the Birck Nanotechnology Center and the Bindley Bioscience Center in Purdue's Discovery Park. The work also is associated with the Purdue Center for Cancer Research and the university's Center for Drug Discovery.
Writer: Emil Venere, 765-494-4709, venere@purdue.edu
Sources: Bumsoo Han, 765-494-5626, bumsoo@purdue.edu
Kinam Park, 765-494-7759, kpark@purdue.edu
Murray Korc, 317-278-6410, mkorc@iu.edu
ABSTRACT
Targeting the Tumor Microenvironment
Kinam Park1, PhD, Bumsoo Han2, PhD, and Murray Korc3, MD 1Weldon School of Biomedical Engineering and 2School of Mechanical Engineering Purdue University, West Lafayette, IN 47907 3Division of Endocrinology, Department of Medicine Indiana University, Indianapolis, IN
Personalized medicine, or precision medicine, relies on the selection of the correct drugs, or drug combinations, based on the disease-specific genetic traits. Selecting the proper drugs is the first step toward precision medicine, but its completion needs effective delivery of the selected drugs to the target (e.g., tumor). Recent progress in nanotechnology has made drug delivery more efficient compared with the control solution formulation, but subsequent effectiveness of the drugs delivered is still in question. Nanoparticulate drug delivery systems are designed and tested for the ultimate goal of developing clinically useful formulations to treat various cancers. Thus, the usefulness of nanoparticle formulations needs to be considered in the context of treating cancers (i.e., improving efficacy and safety) in human patients.

