Testing drugs within a tumor may combat pancreatic cancer drug resistance, ‘time machine’ suggests

Many patients with pancreatic cancer have only about a 10% chance of survival within five years of their diagnosis because they tend to become resistant to chemotherapy, past studies have indicated.
A “time machine” that Purdue University engineers designed to observe pancreatic cancer behavior over time suggests a new drug testing approach that could help scientists better catch resistance.
The researchers found that testing potential drugs on multiple tumor cell subtypes – rather than on just one cell subtype – can reveal drug resistance that may occur due to how different cancer subtypes interact with each other.
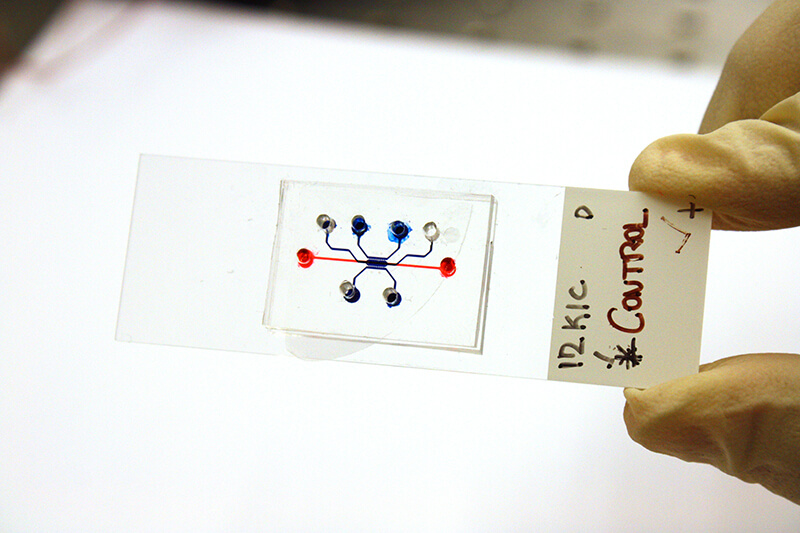
Purdue engineers built a “time machine,” or microfluidic pancreatic tumor device, that simulates tumor growth over time. (Purdue University photo/Jared Pike)
The study was recently published in the Royal Society of Chemistry journal Lab on a Chip.
“The drug discovery and screening process has been using one cancer cell subtype and studying how it interacts with neighboring non-cancer cells, but this may overestimate the efficacy of the drug,” said Bumsoo Han, a Purdue professor of mechanical engineering and program leader of the Purdue Center for Cancer Research. Han has a courtesy appointment in biomedical engineering.
“By condensing time to look at how cancer cells interact within a pancreatic tumor, we found that one cancer cell subtype can not only be more drug-resistant than the others, but drug-sensitive cells can also become resistant through interaction between the subtypes.”
The “time machine” is a type of lab tool called a microfluidic device. These devices are gum strip-sized platforms, such as a chip or slide, where cancer cells can be cultured in channels smaller than a millimeter in diameter. The cells then grow in a lifelike environment on the platform, such as in a collagen tube that Han’s lab created to mimic the pancreatic duct.
Microfluidic devices are starting to become more mainstream in the drug development process because they allow scientists to test drugs in realistic simulations of a biological system using real tissue samples, but on a faster time scale than in animal models.

Han’s group found that about 25% of 2019 research publications indexed by PubMed, a biomedical literature database, had used microfluidic devices as models to study tumors from animals or patients.
But most microfluidic devices just show late-stage tumor growth. With Han’s device, scientists can load in cell lines from an animal model or patient before gene mutation has happened, enabling them to see all stages of tumor progression.
While findings made using microfluidic devices need to be validated in humans before being put into clinical practice, they can still shorten the drug development process by offering new research approaches.
The findings from Han’s device highlight the need for studying interactions between cancer cells.
“Not much research has been done on what kind of interaction happens within tumors, so those mechanisms of drug resistance have been overlooked,” Han said.
These findings are already informing the development of new drug compounds.
Zhong-Yin Zhang, the director of the Purdue Institute for Drug Discovery, is using Han’s microfluidic device to test a compound aimed at blocking an oncogenic process that Zhang’s lab has previously identified as playing a role in cancer development.
The device allows Zhang’s team to evaluate the compound not only for pancreatic cancer specifically, but also on multiple cancer cell subtypes.
“The nice thing about this device is that we don’t have to use as much of a compound to see how well it works,” said Zhang, who is also a distinguished professor of medicinal chemistry in Purdue’s Department of Medicinal Chemistry and Molecular Pharmacology and Department of Chemistry.
A patent has been issued for the microfluidic pancreatic tumor device via the Purdue Research Foundation Office of Technology Commercialization. This research is partially supported by grants from the National Institutes of Health, Purdue Center for Cancer Research and Walther Embedding Program in Physical Sciences in Oncology.
About Purdue University
Purdue University is a top public research institution developing practical solutions to today’s toughest challenges. Ranked the No. 5 Most Innovative University in the United States by U.S. News & World Report, Purdue delivers world-changing research and out-of-this-world discovery. Committed to hands-on and online, real-world learning, Purdue offers a transformative education to all. Committed to affordability and accessibility, Purdue has frozen tuition and most fees at 2012-13 levels, enabling more students than ever to graduate debt-free. See how Purdue never stops in the persistent pursuit of the next giant leap at https://purdue.edu/.
Writer, Media contact: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Sources: Bumsoo Han, bumsoo@purdue.edu
Zhong-Yin Zhang, zhang-zy@purdue.edu