Measuring Visible Light
How can we measure visible light?
The electromagnetic spectrum includes all forms of light energy, known as electromagnetic radiation. (1) Different types of electromagnetic radiation such as radio waves, microwaves, visible light, and x-rays are associated with specific wavelengths. A spectrophotometer is an instrument that performs measurements within the wavelength range of 325 to 1100 nanometers. (2) This range of wavelengths includes visible light, which is the form of electromagnetic radiation that our eyes can detect.

Lesson Overview:
Students will use a spectrophotometer to learn about the wavelengths that correspond with visible light.
Learning Objectives:
- Students will learn about wavelength and use their understanding to observe how visible light can be measured.
- Students will compare observations of visible light to develop wavelength ranges for different colors.
Teachers: Request an Answer Key
Connection to Content Standards, Crosscutting Concepts, Science and Engineering Practices
Key Vocabulary:
- spectrophotometer
- electromagnetic radiation
- wavelength
- visible light
Materials:
- Spectrophotometer
- White paper
- Pen or Pencil
- Student Handout: Procedure
- Data Table and Discussion: Measuring Visible Light
- Google Sheet: Data Analysis: Measuring Visible Light (Teachers: Make a copy of this template to share with students for entering their data.)

Procedure:
- Check that the sample compartment is empty before turning on the spectrophotometer.
- Power ON the spectrophotometer. The power-up sequence will take about 2 minutes to complete. Allow the instrument to warm up for 30 minutes before using it. (3)
- Press A/T/C to select absorbance mode.
- Set the spectrophotometer to a wavelength of 325 nm. (This is the lowest wavelength available for the Genesys 20 spectrophotometer.)
- Press 0 ABS/100%T to set the blank to 0 Absorbance.
- Place a thin strip of white paper in the sample compartment of the spectrophotometer.
- Leave the sample compartment open. Beginning with the wavelength of 325 nm, increase the wavelength until you can first see a color. Record your observation in the chart under the Results section.
- Continue increasing the wavelength and watching the color displayed on the paper strip in the sample compartment. Record the last wavelength that you are able to still observe the first color.
- When you notice a color change, record the new color and the first wavelength of which you observe this new color.
- Continue increasing the wavelength on the spectrophotometer. Record each new color with both initial and final wavelengths observed for each color.
Results:
|
Observed color |
Initial wavelength (nm) |
Final wavelength (nm) |
| Red | ||
| Orange | ||
| Yellow | ||
| Green | ||
| Blue | ||
| Violet | ||
| Indigo |

If you do not have access to a visible spectrometer, you can use the video above for data collection. We recorded the sample compartment while we ran the lab.
Data Analysis:
- Enter the values that you recorded in your Data Table in the Google Sheet: Data Analysis: Measuring Visible Light
- Observe the waterfall chart that is generated as initial and final wavelength values are entered by all groups/participants for each observed color.
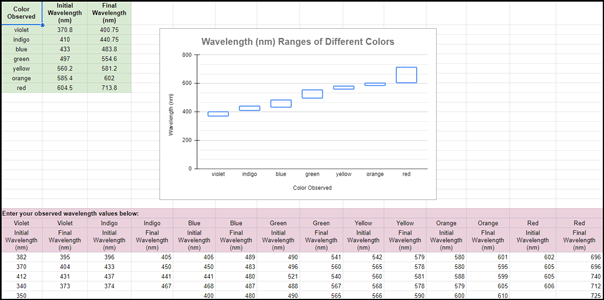
Discussion:
1. How do your observed wavelength values for each color compare to the wavelength values displayed on the graph?
2. Why might your observations of colors at different wavelengths be different from the observations of a classmate?
3. Why do we show individual colors as a range of different wavelengths instead of one single wavelength?
Supplementary Activity: Superheroes of Science Content Expert Video
- Listen or view this Superheroes of Science podcast: Astrophysicists use the electromagnetic spectrum to study stars, pulsars, black holes and more. Complete the guided listening worksheet as you listen to or view the podcast.
- Audio only
- YouTube video
- (Student Handout) Guided Listening - Electromagnetic Spectrum
Additional Resources:
References:
- NASA Goddard Space Flight Center - Imagine the Universe! The Electromagnetic Spectrum https://imagine.gsfc.nasa.gov/science/toolbox/emspectrum1.html
- Thermo Spectronic Service Manual: Genesys 20 Spectrophotometer https://archive-resources.coleparmer.com/Manual_pdfs/genesys%2020%20service%20manual.pdf
- Thermo Spectronic Operator’s Manual: 20 Genesys Spectrophotometer https://archive-resources.coleparmer.com/Manual_pdfs/Genesys%2020%20Op%20Manual%20(2).pdf
NGSS Standards: Measuring Visible Light
(Middle School)
- This standard emphasizes student understanding of wave properties. In learning about the parts of a wave, students will learn about wavelength and use their understanding to observe how visible light can be measured.
(High School)
- This standard emphasizes student understanding of wave properties. In learning about the parts of a wave, students will learn about wavelength and use their understanding to observe how visible light can be measured.
These Crosscutting Concepts and Science and Engineering Practices provide a comprehensive approach to understanding and teaching principles of waves related to the electromagnetic spectrum within the framework of the NGSS.
Crosscutting Concepts:
- Patterns: Observing patterns in data to identify relationships and make predictions. In the context of measuring wavelengths, recognizing the regular patterns in wave properties, such as wavelength, is crucial. (Example: Students use multiple wavelength measurements to generate a waterfall chart that displays the wavelength ranges of visible light.)
- Systems and System Models: Using models to represent and understand systems and their interactions. In wave measurement, models help explain how light waves interact with matter and how these interactions can be measured and analyzed. (Example: The waterfall chart created from student data in this lab allows for a clear, segmented, and visually appealing representation of the continuous nature of visible light.)
- Energy and Matter: Tracking energy transfer and conservation in systems. Measuring wavelengths involves understanding how different wavelengths correspond to different energies. (Example: The data collected from this lab demonstrate that each color of light is associated with a range of wavelength values. These wavelength values are inversely related to the energy values in electromagnetic waves.)
- Scale, Proportion, and Quantity: Using measurements and understanding scale. Accurate measurement of wavelengths requires an understanding of the scale and proportion of the light waves being measured. (Example: Students will note, as they collect wavelength measurements, that the unit of measurement for the wavelengths of visible light is the nanometer.)
Science and Engineering Practices:
- Asking Questions and Defining Problems: Formulating questions that can lead to investigations of light and its properties. (Example: How can we measure the wavelengths of visible light?)
- Planning and Carrying Out Investigations: Designing and conducting experiments to measure wavelengths of light. (Example: Students use a spectrophotometer to measure the wavelengths associated with different colors of visible light.)
- Analyzing and Interpreting Data: Interpreting the data obtained from experiments to determine wavelengths and understanding the implications of these measurements. (Example: Students share measured wavelengths and generate one graph from all of the collected data to understand the ranges of wavelengths associated with each color of visible light.)
- Engaging in Argument from Evidence: Using evidence from investigations to support claims about the properties of light and the accuracy of wavelength measurements. (Example: Students use multiple wavelength measurements to generate a waterfall chart that displays the wavelength ranges of visible light. Because this type of chart can show segments of data, it facilitates easy comparison and understanding of how different wavelengths correspond to different colors.)