Part III: Determining Buffer Components for a Desired pH
This is part of our Virtual Buffers Lab series: Understanding, Making, and Using Buffers
This lab includes a theoretical explanation of how buffers work and how they are made, including a derivation of the Henderson-Hasselbalch equation. Students will use what they have learned to complete a Buffer Challenge.
Learning Objectives
|
Students will be able to… |
|
|---|
Key Vocabulary
- Acid
- Base
- pH
- Strength
- Weak acids and bases
- Strong acids and bases
- Concentration
- Conjugate acid-base pair
- Buffer
Documents
TEACHERS: Request an Answer KEY for this lab activity (Part III), as well as the other three lab activities in this "Understanding, Making, and Using Buffers" virtual lab.
Part III: Determining Buffer Components for a Desired pH
Review of Acids, Bases, and pH
The Brønsted-Lowry definitions for acids and bases are as follows:
- An acid is any substance that can donate one or more protons to another substance.
- A base is any substance that will accept one or more protons from another substance.

Water behaves as both an acid and a base. When water behaves as an acid, it donates one of its two protons and becomes a hydroxide ion.

When water behaves as a base, it accepts a proton and becomes a hydronium ion.

When an acid or base is added to an aqueous solution, it will interact with water molecules to either increase or decrease the concentration of hydronium ions in the solution, which is measured as the solution’s pH.
The “p” in pH can be thought of as “power,” referring to the power of 10 (or order of magnitude) of the concentration. The answer to a log is an exponent, so we can rewrite the pH expression above as:
This equation demonstrates that a higher concentration of hydronium ion will result in a lower pH, and a lower concentration of hydronium ion results in a higher pH.
Factors that affect pH
There are two factors that affect the hydronium ion concentration, and therefore the pH, of a solution: the strength and the concentration of the acid or base in the solution.
The strength of an acid or base affects the pH more significantly than the concentration of the acid or base.
The strength of an acid or base is how likely it is to donate or accept a proton in an acid-base reaction. Strong acids and bases are very likely to donate or accept protons, whereas weak acids and bases are less likely to donate or accept protons.
- For example, hydrochloric acid (HCl) is a strong acid. Essentially, 100% of the protons are transferred to water in an aqueous solution of hydrochloric acid, leaving only chloride anions (Cl – ) behind and drastically increasing the hydronium ion concentration in solution.
- Acetic acid (CH 3COOH), on the other hand, is a weak acid. Only some of the protons will be transferred to water in an aqueous solution of acetic acid, so it does not increase the hydronium ion concentration as much as a strong acid.
- Sodium hydroxide (NaOH), as well as all alkali metal hydroxides, is a strong base. Metal hydroxides will ionize in an aqueous solution, producing a metal cation and the hydroxide anion, which acts as a strong base. Hydroxide ions readily accept a proton to become water.
NaOH <---> Na+ + OH-
- Ammonia (NH3 ) is a weak base. It will accept a proton to become the ammonium ion (NH4+ ), but it does so more reluctantly than other, stronger bases.
The concentration of the acid or base, like all substances, is how many particles are present in a certain volume of a solution. Higher concentrations have a greater effect on the pH than lower concentrations because there are more particles available to react with water and change the hydronium ion concentration.
The purpose of a buffer is to maintain the pH of a solution.
Buffers work to maintain the pH of a solution by reacting with and neutralizing any strong acid or base that is added to the solution. To do this, buffer solutions must contain both a weak acid, to react with strong bases, and a weak base, to react with strong acids.
Why don’t the weak acid and weak base in the buffer solution react with and neutralize each other?
The weak acid and weak base in a buffer solution are a conjugate pair. A conjugate acid-base pair is a pair of chemicals that turn into each other when a proton is gained or lost. For example, when carbonic acid (H2CO3) loses a proton, it becomes the bicarbonate ion, HCO3– , its conjugate base. If HCO3– gains a proton, it becomes H2CO3 again.

Because both the acid and base in a buffer solution are conjugates of each other, the reaction can move in whichever direction it needs to counteract the addition of strong acids and bases and maintain the pH of the solution.
- A strong acid, like hydrochloric acid (HCl), will dissociate completely in a carbonic acid buffer solution, causing the bicarbonate ions to accept the excess protons and form carbonic acid molecules. Carbonic acid molecules are a much weaker acid than the hydrochloric acid. Therefore, the pH rises back to its original range.
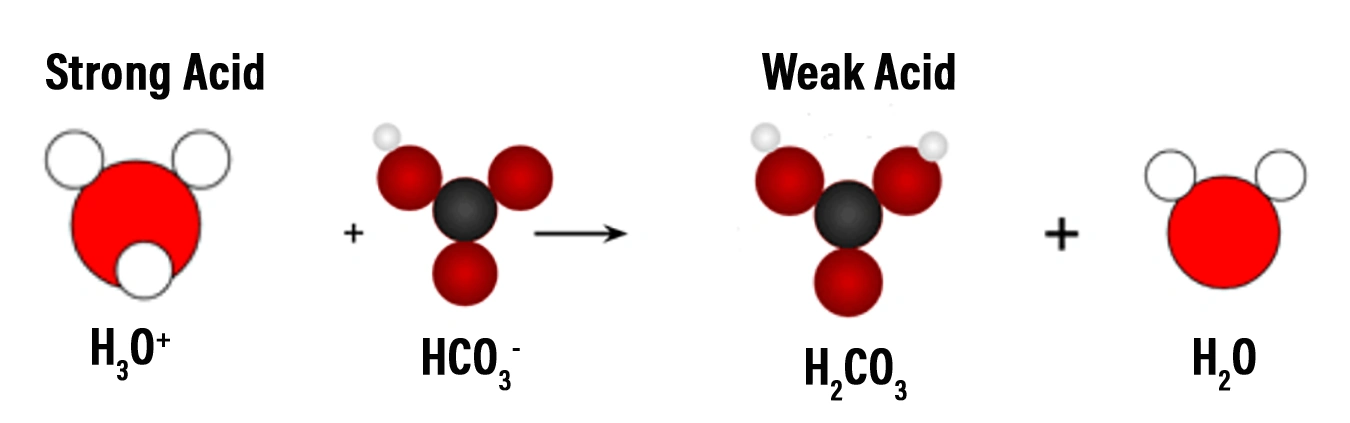
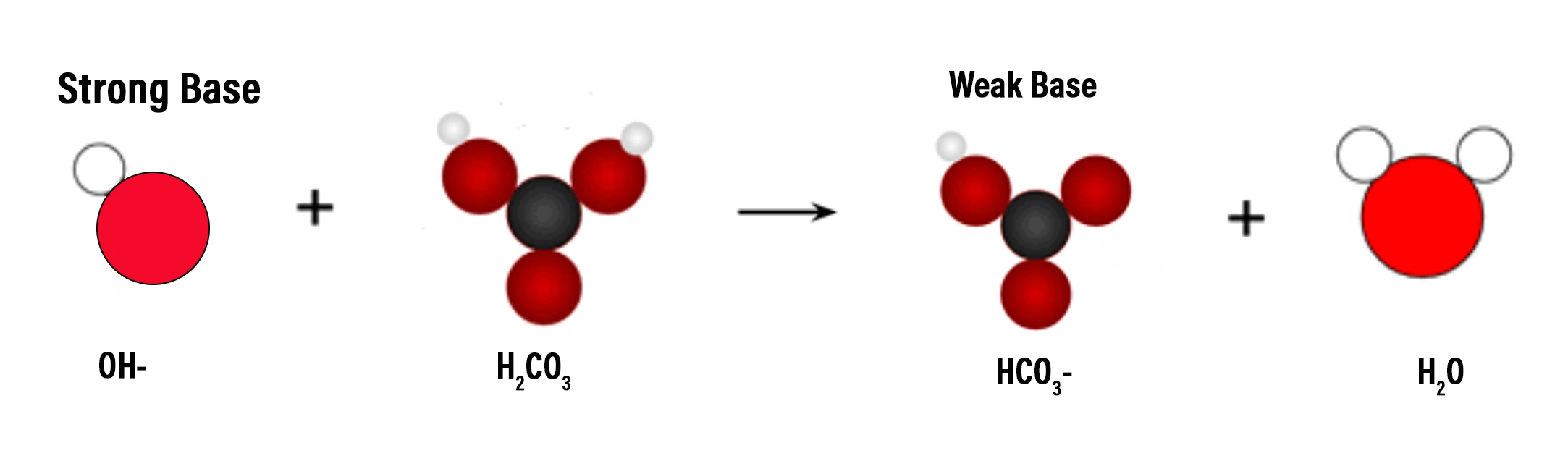
- A strong base, like sodium hydroxide (NaOH), will dissociate completely in a carbonic acid buffer solution. The added hydroxide ions react with carbonic acid, causing the carbonic acid to release protons. The protons released from carbonic acid bond to the hydroxide ions, forming water. This prevents a significant increase in the pH of the solution. The bicarbonate ions produced when the carbonic acid releases protons act as a reservoir of base, ready to react with any additional acid that may be added to the solution.
Determining Buffer Components for a Desired pH
The general reaction for an acid in aqueous solution is:

Recall the strength of an acid is how likely the compound is to donate a proton, which means the reversible reaction above will establish an equilibrium based on the strength of the acidic compound.
The equilibrium constant of a chemical reaction is:
![K=[Products]/[Reactants]](LabImages/buffersp3fig8.1.gif)
For the reaction between an acidic compound and water, the equilibrium constant (or acid dissociation constant) is:
![Ka=[H3O+][A-]/[HA]](LabImages/buffersp3fig9.2.gif)
The concentration of H2O is excluded because it is a pure liquid.
The pH of a buffer solution can be determined by taking the negative logarithm of the equation above.
![-log(Ka)= -log[H3O+][A-]/[HA]](LabImages/buffersp3fig10.gif)
Using the product logarithm rule, the negative log of the concentration of hydronium ion, or pH, can be separated from the rest.
![-log(Ka)= -log[H3O+]-log[A-]/[HA] pKa=pH-log[A-]/[HA]](LabImages/buffersp3fig11.gif)
Finally, recognize the ratio [ A- ] / [HA] as the components of a buffer solution: an acid (HA) and its conjugate anion (A–). This term in the equation is eliminated when the ratio of the components is 1:1 because the logarithm of one is equal to zero.
Given the equilibrium constant of the acid, Ka , the pH range of the buffer solution can be calculated using the equation:

Buffer Challenge
Determine which buffer components from the list should be used for each scenario described below.
- Step 1: Use the Ka values to calculate the pH using Equation 4 (above) for each acid listed.
- Step 2: Choose the appropriate acid for each scenario based on the calculated pH values. Then match the conjugate base to the chosen acid.
- Step 3: Use stoichiometry (setup below) to calculate how many grams of salt need to be added to 100 mL of the aqueous solution of its conjugate to create a 1:1 ratio. (The concentration of all possible solutions are indicated as 0.10 M in Table 1 below. Substances without a concentration of 0.10 M can be assumed to be solid salts.)
![]()
Buffer Components
|
Acids |
Bases |
|---|---|
|
0.10 M acetic acid (CH3COOH)
Ammonium chloride (NH4Cl)
0.10 M citric acid (H3C6H5O7)
0.10 M sodium dihydrogen phosphate (NaH2PO4)
|
0.10 M ammonia (NH3)
Sodium dihydgrogen citrate (NaH2C6H5O7)
Sodium acetate (NaCH3COO)
Sodium hydrogen phosphate (Na2HPO4)
|
Scenario 1: Prepare a buffer for an antibiological agent which is designed for use in the human body. This buffer should have a pH of 7.2 ± 0.5 with the ability to stay within one pH unit of this target when strong acid or base is added.
Scenario 2: Prepare a buffer for an antifungal agent which is designed for use against a fungus that attacks food sources that grow in acidic soil. This buffer should have a pH of 4.7 ± 0.5 with the ability to stay within one pH unit of this target when strong acid or base is added.
Scenario 3: Prepare a buffer for an antifungal agent which is designed for use against a fungus that attacks food sources in basic soil. This buffer should have a pH of 9.2 ± 0.5 with the ability to stay within one pH unit of this target when strong acid or base is added.
Scenario 4: Prepare a buffer for an antiviral agent which is designed for use against a strain of virus that attacks drug-producing bacteria that survive and grow in acidic environments. This buffer should have a pH of 3.1 ± 0.5 with the ability to stay within one pH unit of this target when strong acid or base is added.
 |
 |
 |
This lab was created with support from the Ren Research group at Purdue University with funding from the National Science Foundation grant NSF CHE 2102049.
